Expanders are the essential part of a negative active material which helps in preventing to loose their performance during charge discharge cycle. The loss of performance caused due to passivation which results from deposition of an unwanted layer of Lead Sulfate on the Lead substrate and to loss of porosity caused by shrinkage of Lead sponge.In the Negative plate of Lead acid battery, the reaction occurs with Spongy Lead so the additives who can prevent shrinkage during charge-discharge cycles to improve surface area, are always inevitable.
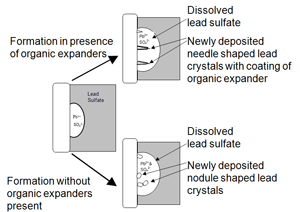
Barium sulfate is electrochemically inactive and extremely insoluble in Sulfuric acid. This property assures that it will be unchanged even during prolonged life cycle. Barium sulfate and Lead sulfate are isostructural and have the same orthorhombic structure. Both have the almost same R value. The single and cation bond of Barium and Lead sulfates are also same.
So all these properties helps to provide a large number of nucleation sites for precipitation of Lead Sulfates crystallization and hence prevents the deposition as a impermeable, thin passivation film. This helps to formate the small crystals of lead sulfate instead of large crystal which are difficult to charge.

More
The improvement herein results from utilizing water soluble and/or water dispersible materials in the steps required producing the paste. By water soluble and/or water dispersible, it is meant a material having at least a 0.5% by weight solubility in water, or a material which readily forms dispersion in water upon simple mixing. It can also mean a material readily "wet" by water such that a material in contact with the water becomes evenly distributed upon mixing. Specifically, water soluble and/or water dispersible forms of barium containing materials are used herein, dissolved in a portion of the water used to make the paste. This water is then mixed with lead oxide powder and other non-soluble/non-dispersible paste materials to form a first mixture. Upon addition of sulfuric acid to this mixture, the barium precipitates as barium sulfate. This barium sulfate formed by this procedure is evenly distributed throughout the paste. As such, the amount of mixing to form a uniform past by this method is less than the mixing required to form a paste from barium sulfate, and other non-water soluble materials that have been added in dry form and then mixed into a paste with liquid. In addition to a more uniform paste, the reduction in mixing required to produce the final negative paste allows for a continuous process of paste formation and application to be used.
The barium source is present in the mixture at a concentration at least about 0.1%, preferably at least about 0.5%, with a concentration at least about 1% based upon the total lead oxide used to form the final paste being more preferred. Also, the barium source is present at a concentration of at most about 10%, preferably at most about 5%, with a concentration at most about 3% based upon the total lead oxide used to form the final paste being more preferred.
Barium sulfate is used in Vectra in two forms according to usage of Batteries. First one is Blanc Fixe which is precipitated from solution and having a median particle size of app 1 microns.

The other one is Barytes which has a median particle size of app. 3.5 microns. They are slow releasing agents but having good dispersion and flow, helped during deep discharges.
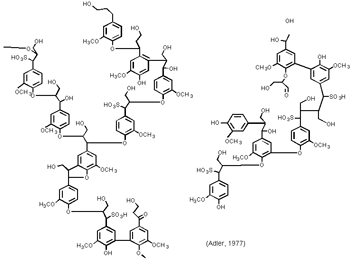
Lignosulfonates are complex aromatic polyethers which have the hydrophobic and hydrophilic anion parts where the hydrophobic Artist�s Representation of Softwood Lignosulfonate Structurepart absorbed on the surface of the lead particles while hydrophilic part of anion facing out to the aqueous electrolyte phase. When the sulfuric acid is mixed with the Lead Oxide during Paste mixing, Different type of Lead sulfate formed which includes Mono basic, tribasic acid and Tetra basic Lead sulfates. Lignosulfonates suppress the formation of tetra basic lead sulfates and Orthorhombic lead Oxide Phrases and helps to create tribasic lead sulfates. This results in an increase in the repulsion potential which prevents the lead particles from sintering or coalescing. So the surface area of Negative active material can improve up to 3-4 times. It is believed that the lignin sulfonic acid, including its synthetically derived analogs, somehow changes the crystal growth of lead sulfate formed during discharge of the battery such that the lead sulfate does not form a continuous film over the surface of the spongy lead. This allows for a discontinuous structure of spongy lead in contact with the sulfuric acid electrolyte, and thus production of electrical energy from the battery. It has also been discovered that without an organic expander, particularly, for example, the sulfonate derivatives of lignin or their equivalent, lead crystals become quite large in diameter and cover the plate and result in low capacity and poor cycle battery life.
More
The organic expander useful herein is either liquid phase or powder phase and is present at a concentration of at least about 0.1%, preferably at least about 0.5%, with a concentration of at least about 1% based upon the total lead oxide used to form the final paste being more preferred. Also, the organic expander is present at a concentration at most about 10%, preferably at most about 5%, with a concentration at most about 3% based upon the total leady oxide used to form the final paste being more preferred.
Suitable sulfonate derivatives of lignin and their synthetic organic analogs will have surfactant properties which allow the lignin derivative to be absorbed onto the spongy lead, as well as on the surface of the lead sulfate crystals formed during discharge. By surfactant properties, it is meant the surface tension of an aqueous solution of the material is lower than water alone. It is believed that these properties inhibit crystal growth during the dissolution-precipitation process.
This results in more contact of lead metal with the electrolyte so that the current per unit area of electrodes become lower and reduce the effective current density during discharge and thereby increase in active material utilization. Hence the high rate discharge or longer duration can be obtained.
Lignin exists in different type like Oxy lignin, Kraft lignin and synthetic lignin (derived from treatment with formaldehydes) with different salts inclusive of Sodium, calcium and ammonium. it has been found that both the cycle life and the cold crank ability of a storage battery of the lead-acid type can be improved beyond using the oxylignin or Kraft lignin alone by incorporation of a mixture (or reaction products) of both the oxylignin and the Kraft lignin. In effect there appears to be a synergy between the two materials. When used together in an appropriate blend the result is improved cycle life and cold crank ability performance beyond either when used alone.
Different group and property of a typical Lignosulfonates like, Methoxy, carboxyl, Ketonic, Phenolic and Hydroxyl, also affect on the capacity and life cycle of a Lead acid Battery. Reducing Sugar and purity of lignin also plays a major role in performance. Researches have correlate expander performance with the content of different structural groups and the molecular weight of lignosulfonates. They found that the strongest beneficial effect on battery performance was obtained with materials having low average molecular weight. They also found a correlation between capacity and the content of carboxyl groups in the molecule. High carboxyl contents favored improved capacity and a reduced rate of self discharge. Increasing the percentage of methoxy groups in the molecule had an adverse effect on cold cranking behavior while increasing the organic sulfur content increased the rate of self discharge. A complex relationship was found between the percentage of phenolic groups and the cycle life with negative effects on self discharge and charge acceptance and a positive effect on cycle life.
The effect of lignosulfonates on the recharge behavior of negative plates is well known and it has been observed that various organic materials affect the charge acceptance and the polarization in different ways. This may require each lignosulfonate to have a unique charge algorithm to optimize its performance. In the present work, all cells were charged with the same algorithm and no attempt was made to optimize the recharge behavior. It is possible, therefore, that different cycle lives would have been obtained with a different charge algorithm. These problems are not seen in flooded cells since oxygen recombination does not occur and the negative plate is sufficiently polarized to ensure its full charge. The cycle lives obtained from these materials therefore may have been considerably different in flooded cells.
Scanning electron microscope (SEM) images for several of the additives have proved that without an organic additive the NAM is composed of dense overlapping shingle-like crystals with low porosity. All of the organic additives changed the morphology to a small equiaxial crystal form with increased porosity. In good agreement with the surface area studies, those additives that gave the greatest surface area also created the finest grained, most porous structure. Comparison of the plates at different cycles showed little change in morphology, however, there were indications of loss of surface porosity compared to the interior. At the end of life, the SEM images of the active material had become denser and contained a considerable amount of lead sulfate. The large size of these crystals is consistent with slow growth giving support to the idea that self discharge is responsible for at least part of the capacity loss during cycling.
Carbon Increases the conductivity of negative active material to assist in the initial charging of battery and during deep discharge where concentration of highly resistant of Lead sulfate is high. Induction of carbon increases the life cycle of lead acid battery while the expanded graphite improves the conductivity of lead acid battery. These carbons also increase the speed of formation during the curing of Battery plates. Carbon is considered an oxidation source in the battery, after the plates are formed, carbon black is likely a detractor from the life of the battery.
More
The different form of carbon i.e. Granular and powdered carbons are used according to the usage of Lead acid Battery. The structure of the primary aggregates, which do not break down, determines the properties of each grade of black. Low structure blacks have few primary particles in compact structures. High structure blacks have many primary particles fused into long, complex chains. The combination of particle size, structure, and surface oxidation determine the properties such as colouring ability, UV absorption, and conductivity.
Small quantity of Graphite enhances the formation by increasing the conductivity of the paste. It also promotes the formation of alpha particles of PAM, which results in better coarseness and porosity. It also helps in the decreasing of gas evolution on the initial stage. Graphite helps in increasing the capacity & initial utilization of NAM and PAM.
Sodium sulfate is not isomorphous with lead sulfate and is more soluble. When SS used as an additive it decreases the solubility of lead sulfate due to common ion effect which result in reducing the number of failure from shorting when the battery is deeply discharged. SS increases utilization of material by dissolving to create a more porous structure in the plate. A very less quantity of SS in the plate can improve the higher initial capacity and average capacity per cycle.
These dispersants have the ability to stabilize and to reduce the viscosity of high solids aqueous inorganic slurries of the type contemplated. The preferred dispersants are anionic polynuclear aromatic compounds which have a hydrophilic backbone which is adsorbed onto the surface of the suspended lead or lead-oxide particle. This is believed to impart a negative charge to the suspended particle which then repels one another. The lessening of the attractive forces between particles aids in breaking up flocks or agglomerates and forces each particle to act as a single unit. The result is a true dispersion, containing particles of optimum fineness with no appreciable agglomeration.
More
Unlike surfactants, these dispersants of invention do not lower surface or interfacial tension and, hence, they have little or no tendency to create foam. The most preferred materials are synthetic naphthalene sulfonic acid condensates, most preferably formaldehyde naphthalene sulfonic acid condensates. Although not narrowly critical, these condensates have an average number of monomer units in the range from about 6 to about 50 (i.e. a molecular weight from about 1,450 to about 12,150), more preferably a monomer unit average of from about 7 to about 12, most preferably 9. The sulfonated aromatic dispersant is believed to function to improve dispersion of barium sulfate in the paste, reduce hydroset time, and produce a stronger plate which is resistant to plate breakage, to reduce fine lead particles and thereby improve handling and to improve pasting characteristics. It extends the life of the battery by increasing the end of charge voltage.
To keep the lead and lead oxide solids suspended in the liquid vehicle, nonionic thickeners, notably hydroxypropyl cellulose being used as a processing aid to increase slurry fluidity by reducing its viscosity, and to retard settling. The hydroxypropyl cellulose solution foams. Furthermore, the hydroxypropyl cellulose thickener in solution undergoes acid catalyzed hydrolysis and the solution is slightly acidic (with sulfuric acid) to minimize transformation of massicot (yellow, rhombic lead oxide) to litharge (red, tetragonal lead oxide). Hydrolysis of the hydroxypropyl cellulose thickener leads to solutions of lower viscosity (lower molecular weight), complicating process control.
More
Furthermore, cells made with hydroxypropyl cellulose thickener as a processing aid are retained within the cell, and may degrade cell performance since at least the carbon black portion of the expander e.g. including barium sulfate and lining sulfonate, does not appear to be uniformly dispersed in the paste slurry. Additionally, the hydroxypropyl cellulose thickener does not permit achievement of sufficiently high solids content in the paste as desired, resulting in producing negative plates in particular which are lower in bulk density than desired. The high discharge rate performance of cells made with hydroxypropyl cellulose thickener also appears to be impaired.
It is an object of this invention to produce electrode plates for lead-acid cells by applying slurry-type suspensions onto a suitable substrate using a processing aid which permits extrusion of a dense but fluid (workable) slurry onto a suitable substrate.
The lead-acid Battery has different characteristics that make it attractive for many applications. These include high specific power, power density &volumetric energy Density. However, lead Battery has low specific energy i.e. Energy per unit weight. The major region for this limitation is that much of the active material is not fully discharged. Therefore, there is always a requirement of using conductive and non-conductive additives in different doses, which results to improve the performance of active material during charge discharge cycle. A typical positive active material has a blend of conductive & non-conductive materials .The non-conductive additives have the larger size then the LeadOxide and are effective in displacing Active Material without seriously decreasing the critical volume fraction. On the other hand the conductive additives have the small particles with the LeadOxide particles.
CarboxyMethylCellulose is used as a pore-forming agent in Positive Active Material. It is an excellent plasticizer and helps to bond individual lead grains.
Titanium Oxide is a conductive additive used as a current collector in Lead Acid battery. The Honey Combed structure of TitaniumOxide holds the paste and thereby improve the paste adhesion and the electrical conductivity as well as the mechanical stability of pasted plate TitaniumOxide is also stable at the potentials of the positive plate.
It improves the capacity during charge discharge cycles and improves the plasticity and binding of Positive Active Material. it results in to improve life cycle, capacity and low temperature performance of Lead Acid Battery.
when we induct the sulfuric acid in a flooded lead acid battery, in stagnant battery, after some time the concentration of Sulfate ions increase in the lower side of cell as compare to the upper side of cell. this un equilibrium results in the earlyshedding of active material .A very little quantity of Fumed silica can make equilibrium in the concentration of sulfuric acid results in better life cycle of Battery.
Sodium Bentonites works as a binding agent. It has a natural swelling ability and maintain its ability to maintain throughout its life.
The major reason for the inability of a lead acid battery to achieve higher specific free energy values is that much of the active material in both the positive and the negative electrode is not discharged. Using additives,hollow glass microspheres, in the electrodes would improve the utilization of active material and could significantly increase the specific energy of the lead acid batteries.
Calcium sulphate added to the positive material of flat or tubular plates of lead/acid batteries significantly improves performance at high rates of discharge, particularly at low temperatures. Calcium sulphate is added to the paste mix (flat plates) or blended with the precursor oxide (tubular plates). Cycle life is unaffected, as seen by the maintenance of the structural integrity of the plates. When the tubular electrodes were cycled, the capacity of the ground positive active- material was gradually restored. Cycle- life increased as a function of the content of calcium sulfate: the most stable capacity was with 0.8wt. % calcium sulfate. It was seen that the addition of calcium sulfate increases the rate of red sulfate nucleation during discharge and modifies the B-PBO2 and lead sulfate structure .The positive active-material contain region of crystalline lead dioxide, as well as regions of amorphous, hydrated, lead oxide called Gel. The calcium ions increase the gel: crystal ratio in the positive active-material and act as binder for the skeletal structure of the material. Another investigation found that calcium sulfate in paste improves the cold cranking ability of automotive batteries.
More
Two types batteries were tasted, namely, a standard 12-v, 60-ah unit a tubular, 8-v, 450-ah diesel electric locomotive battery. Either 0.25 or 2wt. % calcium sulfate was dry blended with the oxide before acid and water additions. The automotive batteries were tested at the C\20 rate and also discharged at high rate at 0 and -15oC. The tubular batteries were discharged at 2300A for 15s, followed by a rest period for 15s. Calcium sulfate increased the voltage at all concentrations and discharge rates, and the effect was more pronounced at higher currents and lower temperature. Lead acid batteries typically show a sharp voltage drop at beginning of discharge, and this is followed by a voltage increase. The phenomenon is called the �Coup de foute� in the above study, the voltage drop was not reduced by calcium sulfate and the voltage increase was only seen after the first 5s of discharge, except under a high rate of discharge at -15oc .
Copyright © 2022 Saraswati Auto Allied. All Rights Reserved || Powered by : Konnect2india